BACKGROUND
Chronic volume overload (VO) is a primary factor responsible for the excessive cardiovascular morbidity and mortality in hemodialysis (HD) patients. VO is caused in part by excessive fluid intake that is secondary to the consumption of a high salt diet. HD patients are often counseled to restrict their dietary sodium intake to help manage thirst and reduce their interdialytic weight gain (IDWG). However, data from recently published investigations demonstrate that dietary counseling alone may be ineffective. The objective of this randomized controlled trial is to determine if short-term feeding of low-sodium meals can “prime” changes in long-term nutrition behavior. It is hypothesized that feeding low-sodium meals for two months will significantly reduce IDWG and related outcomes, and continued dietary counseling and education support for 6 months will result in a sustained reduction in sodium intake upon patient resumption of meal responsibility. HD patients will be recruited and randomized to 2 groups: 1) Low-sodium meal feeding plus dietary counseling; or 2) a waitlist control group that will initially receive dietary counseling alone. IDWG will serve as the primary outcome with fluid volume overload, intradialytic hypotension, cramping, dietary sodium intake, sodium taste sensitivity and preference, and sodium self-efficacy evaluated at 1 and 6 months. These outcomes of this investigation will provide the first data on whether meal provision is an effective tool for dietary modeling and prolonged behavior change in HD patients.
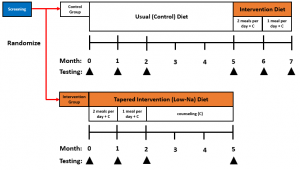
Study Overview
For the two months of home-meal delivery, the meals will be delivered in a tapered manner. During the first month, participants will receive 2 meals per day. They will then receive 1 meal per day in the second home-delivery month. Meals are standardized to <700mg sodium each and meet the National Kidney Foundations (NKF) recommendations of low potassium and phosphorus.
Eligible participants will be randomized to one of two groups:
- Control group (CON; 7 months total): This is a wait-list control group in which participants undergo 5 months of usual care (no counseling or meal provision) followed by 2 months of home-delivered meals OR
- Intervention group (INT; 5 months total): Participants in this group will receive home delivered meals for the first two months of the study, as well as receive additional counseling on how to limit sodium in their diet for the duration of the five month intervention period.